Top Links
Journal of Case Reports and Studies
ISSN: 2348-9820
Lethal Dance with Dialysis – A Case of Silent Long QT Syndrome
Copyright: © 2015 Gorecka M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Fatal arrhythmia is the leading cause of mortality in chronic haemodialysis patients. Long QT syndrome is responsible for polymorphic ventricular tachycardia known as Torsade de Pointes. Classically, long QT syndromes were divided into congenital and acquired; however ‘silent’ variants, in which patients remain asymptomatic until exposed to a drug or electrolyte disturbance precipitating the arrhythmia, have now been recognized. Management of these cases may prove especially difficult in situations where exposure to the precipitating factor cannot be avoided, such as in chronic haemodialysis patients. We present a case of a silent long QT syndrome which resulted in multiple cardiac arrests upon exposure to dialysis and required an insertion of implantable-cardioverter defibrillator.
Keywords: Torsade de Pointes; Dialysis; Long QTc interval; Implantable-Cardioverter Defibrillator
Long QT syndrome is a relatively modern disease, as the first electrocardiographically proven case dates back only to 1935. It is associated with malignant arrhythmias, which were initially called paroxysmal ventricular fibrillation. Only in 1966, the term Torsade de Pointes was coined to describe this polymorphic ventricular tachycardia [1]. There are multiple risk factors associated with prolongation of QTc interval. Among them are medications, especially antiarrhythmic (mainly class I drugs such as Procainamide and Disopyramide; Amiodarone to lesser extent), antidepressant and antipsychotic drugs, but also electrolyte disturbances such as hypokalaemia and hypomagnesaemia [2,3]. Classically, Long QT Syndromes (LQTS) were divided into congenital and acquired; however ‘silent’ variants, in which patients remain asymptomatic until exposed to a drug or electrolyte disturbance precipitating the arrhythmia, have now been recognized. Those patients have a subtype of LQTS and may remain asymptomatic for years until exposed to an offending factor. The underlying cause for presentations such as this may be a true acquired LQTS, or may represent undetected genetic alterations that reduce repolarization reserve in the setting of a drug or environmental challenge [4]. In the modern world, prevalence of patients requiring dialysis and patients requiring antidepressant or antipsychotic medications is quite significant. On average 1.3 million people worldwide are receiving dialysis [5] and the annual growth rate is around 8% [6]. Depressive disorders account for the 4th leading cause of disease burden globally [7]. We believe that this case highlights the importance of careful assessment of patients before and during therapy with QT prolonging medications or electrolyte disturbing interventions, such as haemodialysis.
A 71-year-old man with history of chronic kidney disease, presented for an elective coronary artery bypass graft (CABG) to his right coronary artery (RCA) and mitral and tricuspid valve repair. He suffered from shortness of breath on exertion (100 yards) for the past year due to severe mitral regurgitation. He had become increasingly fatigued during that time, but denied orthopnoea, paroxysmal nocturnal dyspnoea, chest pain, palpitations, dizziness or syncope. He began dialysis immediately before his surgery as a pre-emptive intervention. He underwent mitral and tricuspid valve repair with single graft to RCA without immediate complications. He continued to have acute haemodialysis every 2nd day post surgery. He suffered 3 cardiac arrests – on day 15, 18 and 20 post the surgery. Each of the cardiac arrests was preceded by haemodialysis. His past medical history was significant for chronic kidney disease (congenital solitary kidney), atrial fibrillation, hypertension and depression but negative for palpitations or syncope. His medications included citalopram and trazodone, which he had been taking for the past 15 years as well as valsartan, omeprazole, verapamil, warfarin, ezetimibe and simvastatin. His father died suddenly at age of 65 from a presumed acute coronary syndrome, but there was no other history of sudden cardiac death. He has no siblings and no children. At the time of admission he lived with his wife and was independent of activities of daily living but limited by exertional symptoms.
Baseline blood investigations and repeated Electrocardiograms (ECG) were performed on admission as well as at each of the cardiac events. He had a Transthoracic Echocardiogram (TTE) prior admission and twice post operatively. He also underwent coronary angiography in preparation for valve surgery. The pre-operative TTE showed ejection fraction of 50% with moderate to severe mitral and tricuspid regurgitation. Coronary angiography showed 50-75% stenosis of the RCA. On admission his potassium and corrected calcium levels were normal (4.8 and 2.2 mmol respectively). Magnesium level wasn’t performed. His ECG on admission revealed atrial fibrillation with rate of 63 beats per minute (bpm) and QTc of 433 ms (Figure 1). He was bradycardic post operatively and was started on continuous cardiac monitoring to assess for pauses. Telemetry revealed junctional bradycardia and normal QTc of 444ms. His first Torsade de Pointes (Figure 2) arrest occurred 2 hours post dialysis on day 15 post surgery. After return of spontaneous circulation his ECG revealed atrial fibrillation with a prolonged QTc of 519 ms. His potassium level was normal at 4.3 mmol; magnesium and corrected calcium levels were mildly reduced at 0.79 mmol and 2.13 mmol, respectively. Telemetry continued to show self-terminating runs of ventricular tachycardia. On day 18 he suffered 2nd Torsade de Pointes arrest 3 hours after another session of haemodialysis. His potassium, magnesium and calcium levels were normal. On day 20 post operation, he had 3rd cardiac arrest 10 hours post haemodialysis. His QTc at this stage was almost 600 ms (Figure 3) and all his electrolytes were normal. The latter 2 arrests were preceded by slow atrial fibrillation. There were no pauses.
Initially, post first Torsade de Pointes arrest, his antidepressant medications, which could have been contributing to QTc prolongation, were changed by the psychiatric team to sertraline and lorazepam. He also received intravenous supplementation of magnesium, potassium and calcium as the levels of these electrolytes were mildly low (Mg 0.79, K 3.4, Ca 2.14). During each of his 3 cardiac arrests, he received boluses of amiodarone; however this may have contributed to further QTc prolongation. As his underlying rhythm was slow atrial fibrillation, he was started on isoprenaline infusion to increase his heart rate and thereby reduce the QTc interval. Beta blockers were not introduced due to bradycardia and intermittent 2:1 atrio-ventricular block. Subsequently, he suffered his third Torsade de Pointes arrest with QTc of almost 600 ms. At this stage a temporary pacing wire was inserted and set to a rate of 100 bpm. Isoprenaline was discontinued. Normal QTc interval on admission (433 ms) posed a dilemma regarding this patient’s long-term management, but due to the likely need for ongoing dialysis (eGFR 11 ml/hr), it was decided to proceed with insertion of an Implantable Cardioverter-Defibrillator (ICD). Previous history of a prolonged QTc interval (pre-assessment ECG: QTc 508 ms; atrial fibrillation rate 84 beats per minute) prior to admission supported this decision - despite the lack of history of syncope. Following insertion of the ICD, he underwent haemodialysis and subsequently received 5 appropriate shocks from the ICD in the space of an hour. ICD interrogation revealed QTc of 600 ms and was reprogrammed to VVI mode with rate of 105 bpm. He also received another dose of intravenous magnesium sulphate (Mg 0.79). At this stage he had no further cardiac events and it was agreed that dialysis would be avoided for as long as possible, as the patient had good urinary output and his renal function began to improve. The pacing rate was slowly reduced to 80 bpm and finally to 60 bpm. He has remained asymptomatic.
On subsequent ICD interrogations, the QTc interval remained prolonged (579 ms), but there were no further episodes of VT and the patient remained well. There has been no need for further dialysis as yet. He continues to take sertraline with a satisfactory effect.
Genetic testing was not carried out as this patient had no siblings and no children. Due to the possibility of chronic haemodialysis and recurrent exposure to offending factors, ICD was deemed to be the safest option regardless of the primary pathology.
He is being closely followed up by nephrology and cardiology with regular ICD assessments.
Torsade de Pointes (TdP) is a form of malignant arrhythmia, which may cause sudden death. It is classically precipitated by prolongation of the corrected QT interval on an electrocardiogram. The prolongation may be acquired or congenital. As mentioned before, there are multiple causes of acquired Long QT Syndrome, which include electrolyte abnormalities, such as low potassium, magnesium or calcium, drugs and profound bradycardia [8].
The arrhythmia in the Long QT Syndrome is caused by a dysfunction of ion channels, which in turn impair repolarization of the ventricles [4]. In a healthy heart there are a number of mechanisms to achieve normal repolarization. Usually a malfunction or lesion in only one of them may be insufficient to produce clinically significant impairment of repolarization. Multiple lesions caused by various environmental risk factors, may lead to a reduction in ‘repolarization reserve’. However, not all individuals with a prolonged QTc interval will experience symptoms. It is suggested that genetic predisposition plays a significant role [1].
The main mechanism by which TdP occurs is an early afterdepolarization in the Purkinje fibers. This happens as a result of either reduction of outward repolarizing K current, increase in Na or Ca depolarizing inward current, or the combination of both [9].
Mutations in the genes coding for IKs channels may frequently result in LQTS. IKs potassium channel is activated slowly, which plays a crucial role in tachycardia as it shortens the action potential and allows complete repolarization [10]. Drugs which act on beta-adrenergic receptors, such as isoproterenol will lead to over activation of IKs channels versus IKr and IKi [11]. This property is frequently used in patients with symptomatic prolongation of QT interval, where by administration of isoproterenol heart rate is increased and full repolarization allowed to occur.
The prevalence of cardiovascular disease is significantly higher in patients with chronic kidney disease. Multiple pathologies, such as coronary artery disease, cardiomyopathy, hypertension and left ventricular hypertrophy lead to acquired channelopathies by alteration of cardiac ion channel expressions. This results in reduction of the repolarization reserve and increased risk of arrhythmias [12]. In this predisposed group of patients, administration of medications, which block the IKr channel results in further prolongation of the action potential, which, by a series of events at a cellular level eventually lead to polymorphic ventricular tachycardia [1]. Therefore, it is vitally important, that those drugs should either be avoided or the doses should be adjusted in patients with renal impairment [12].
The prevalence of Sudden Cardiac Death due to an arrhythmia is higher in End-Stage Renal Disease patients, than in any other population. In many cases it is associated with haemodialysis. It is postulated, that during haemodialysis, there is an occurrence of a transient intracellular hypokalaemia and/or hypomagnesaemia. Furthermore, that brief intracellular hypokalaemia is undetectable at serum level and thus measurement of serum level of potassium is of little use [12].
Treatment of acquired long QT syndrome is somewhat controversial. Immediate treatments are focused on stabilization of the myocardium and maintenance of sinus rhythm by supplementation of electrolytes, especially magnesium, but also correction of hypocalcaemia and hypokalaemia if present. In certain cases temporary pacing may be required. In the long-term, patients with a congenital form may be managed by insertion of an ICD, however this is usually not required in patients with acquired syndrome, as in most cases there is resolution of the prolonged QTc, once electrolyte abnormalities have been corrected and offending medications withdrawn. [8] End Stage Renal Disease patients are a vulnerable subgroup of patients, as they are exposed to recurrent electrolyte abnormalities associated with maintenance haemodialysis. Use of an ICD is recommended in those cases and has been shown to significantly increase survival [13,14].
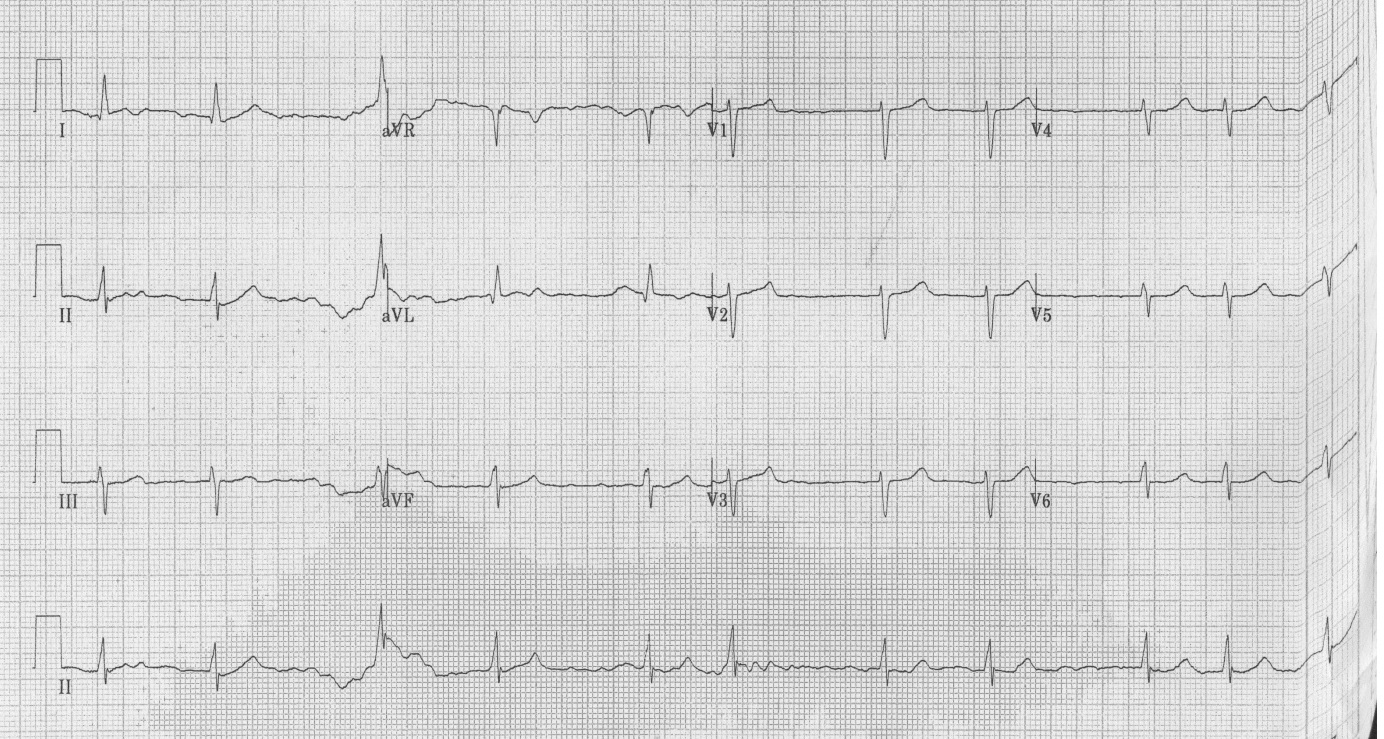 |
| Figure 1: ECG on admission - QTc of 433 ms |
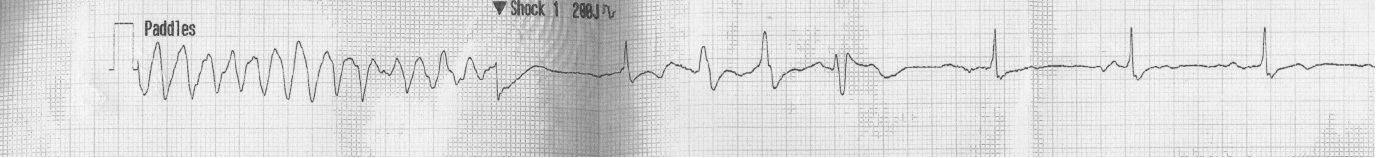 |
| Figure 2: 1st arrest rhythm strip – Torsade de Pointes |
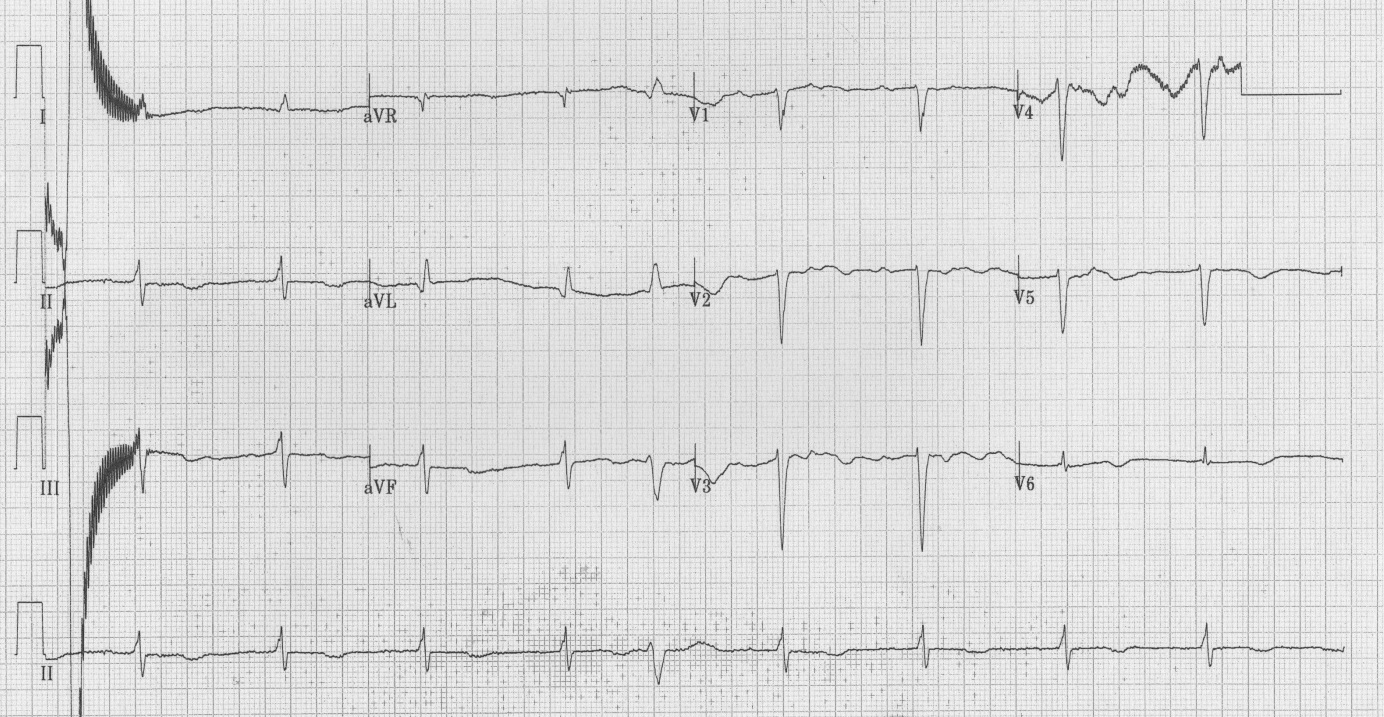 |
| Figure 3: ECG post 3rd arrest – QTc of 598 ms |






































